Indicate the Two Statements That Describe Chemical Bonds.
Although this form of bond is weaker and has a smaller density than a double bond and a triple bond it is the most stable because it has a lower level of reactivity meaning less vulnerability in losing electrons to atoms that want. Atoms always bond by sharing electrons.

Periodic Trends Electronegativity Ionization Energy And Atomic Radius Ionization Energy Chemistry Worksheets Classroom Tools
SYI1 EU SYI1B LO SYI1B1 EK Chemical bonds hold molecules together and create temporary connections that are essential to life.

. A single bond is when two electrons--one pair of electrons--are shared between two atoms. It is a type of chemical bond that generates two oppositely charged ions. Regular Regular Insulin a grid-like arrangement of the positively charged metal ions in a space.
Add octet electrons to the atoms bonded to the center atom. It is a form of chemical bonding between two non-metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. Which statement is true of covalent bonds.
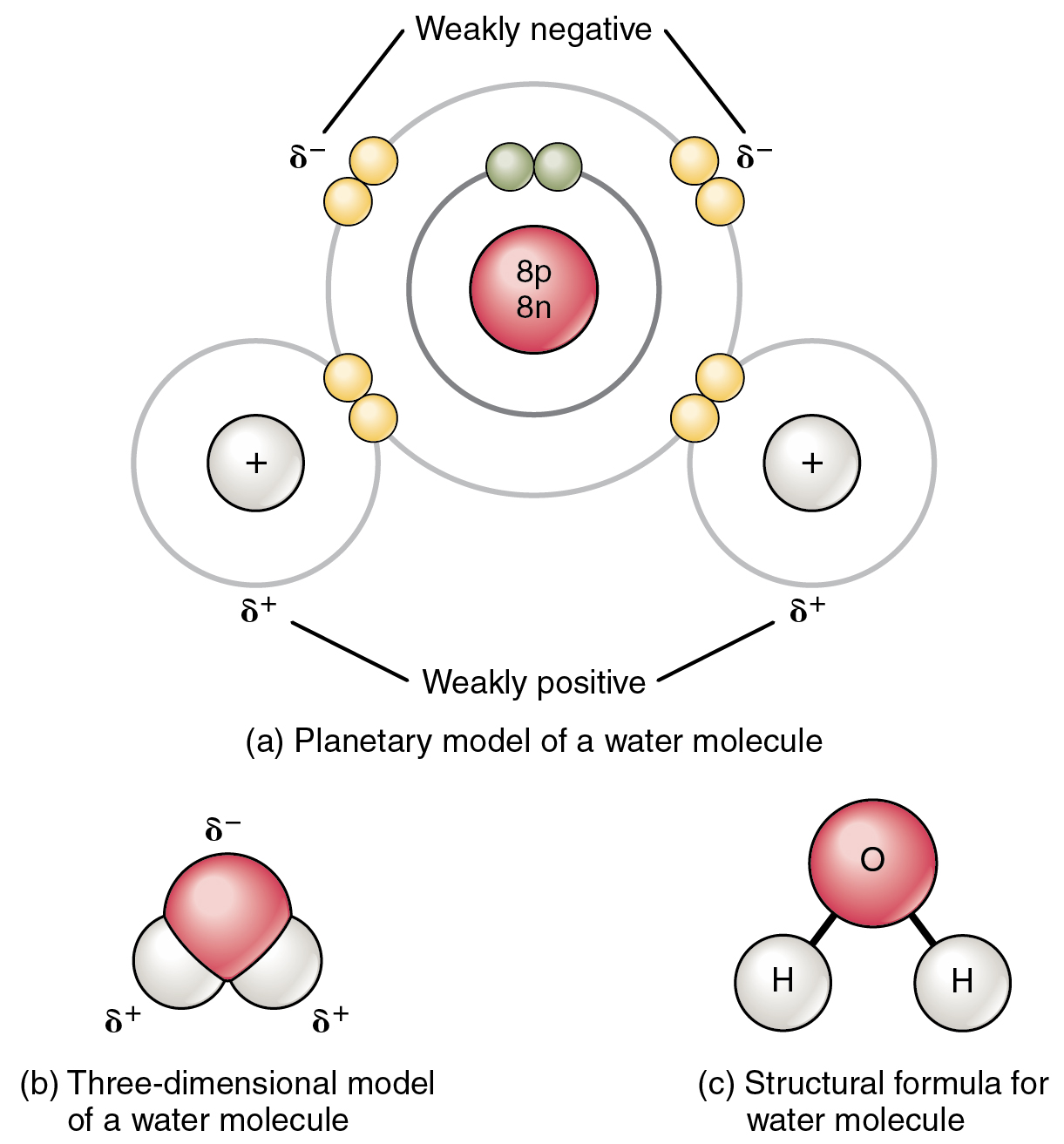
Also known as an electrovalent bond it is a type of bond formed from the strong electrostatic force of attraction between oppositely charged ions in a. In contrast each oxygen atom is bonded to two H atoms at the shorter distance and two at the longer distance corresponding to two OH covalent bonds and two OH hydrogen bonds from adjacent water molecules respectively. Ionic bonds require an electron donor often a metal and an electron acceptor a nonmetal.
A chemical bond produces a. Draw the bond connectivities. Add a multiple bond first try a double bond to see if the central atom can achieve an octet.
The strength of chemical bonds varies considerably. A triple bond consists of one σ bond and two π bonds. Place any leftover electrons 24-24 0 on the center atom.
Select all that apply A chemical bond leads to increased stability. Which of the following statements correctly describe a chemical bond. A stable covalent bond is formed through nucleus-electron attractions.
This force is of an electric nature and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bondsAlthough electrons of one atom repel electrons of another the repulsion is relatively small. -Bond strength increases with the number of electron pairs shared between two atoms. An unstable nucleus that emits particles or energy A force that attracts one atom to another such as their opposite charges or the sharing of electrons A covalent ionic or hydrogen bond A form of an atom with a different number of neutrons Do you know the answer.
-A multiple bond arises when two atoms share two or more electron pairs. Learn about types of bonds and Single bond BYJUS. The substance class can be derived from this binding type.
Ions and Ionic Bonds. A σ bond is stronger than a π bond due to greater overlap. There are strong bonds or.
A triple bond forms when three electron pairs are shared by a pair of atoms as in carbon monoxide CO and the cyanide ion CN. Check all that apply. Atoms bond to attain a full outer shell of valence electrons.
Polar covalent-electrons are being shared unequally between atoms. Does the central atom have an octet. Terms in this set 64 Match each type of bond to the disposition of valence electrons between atoms.
C Each of the two π bonds is formed by overlap of a 2p orbital on carbon and a nitrogen 2p orbital. Covalent bonds form when the. Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion forces.
A double bond forms when two pairs of electrons are shared between a pair of atoms as between the carbon and oxygen atoms in CH 2 O formaldehyde and between the two carbon atoms in C 2 H 4 ethylene. These are ionic bonds covalent bonds and hydrogen bonds. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds.
Chemical bond refers to the forces holding atoms together to form molecules and solids. If the two atoms in a covalent bond approach too closely they will repel each other. It is depicted by a single line between the two atoms.
Instead each hydrogen atom is 101 pm from one oxygen and 174 pm from the other. In ionic bonds the metal loses electrons to become a positively charged cation whereas the nonmetal accepts those electrons to become a negatively charged anion. A large amount of energy 436 kJ must be added to break the bonds in one mole of hydrogen molecules and cause the atoms to separate.
No matter the element there is the same bond length between neighboring atoms. A b The terminal carbon atom uses sp 3 hybrid orbitals while the central carbon atom is sp hybridized. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds.
Single Bond Double Bond Triple Bond - In single bond 2 electrons are shared in double bond four electrons are shared and in triple bond six electrons are shared. The metallic bond is a type of chemical bond that is based on the forces of attraction between positively charged metal ions and negatively charged versatile ions. Indicate the two statements that describe chemical bonds.
Three types of chemical bonds are important in human physiology because they hold together substances that are used by the body for critical aspects of homeostasis signaling and energy production to name just a few important processes. A double bond is a chemical bond between two chemical elements involving four bonding electrons. It is essential to remember that energy must be added to break chemical bonds an endothermic process whereas forming chemical bonds releases energy an exothermic process.
In the case of H 2 the covalent bond is very strong. Does the central atom have an octet. Valence electrons must be shared equally between atoms in order to achieve stability.
Ionic bonding is observed because. NO it has 6 electrons. Ionic-electrons have been transferred from one atom to another.
Pure covalent-electrons are being shared equally between atoms. As two atoms form a covalent bond the stability of the system increases.

Elements Compounds Mixtures Worksheet With Answer Key Persuasive Writing Prompts Scientific Method Worksheet Free Chemistry Worksheets

No comments for "Indicate the Two Statements That Describe Chemical Bonds."
Post a Comment